Question Video: Calculating the Heat Energy Transferred to Water Using Its Specific Heat Capacity | Nagwa

Thermochemistry. Energy and Heat Energy = the ability to do work, measured in Joules (J) 1 joule = 1 Newton of force applied to a 1 kg object over the. - ppt download
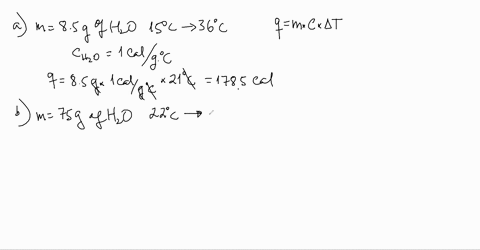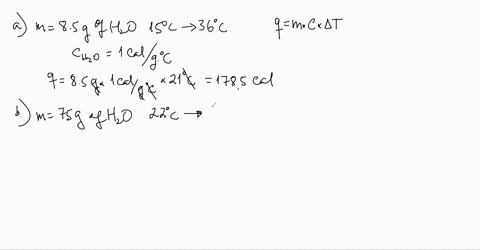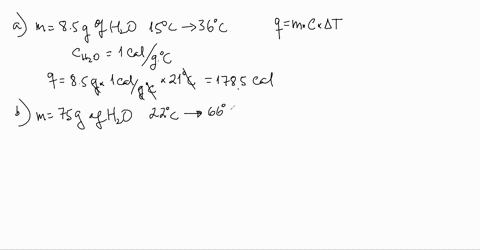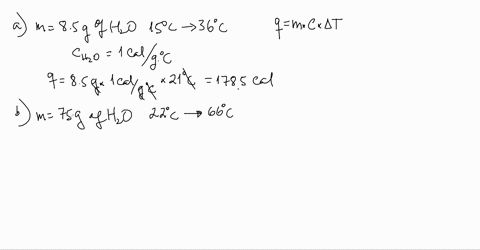
SOLVED:Use the heat equation to calculate the energy for each of the following (see Table 3.11): a. calories to heat 8.5 g of water from 15^∘ C to 36^∘ C b. joules